|
Name
|
Flags
|
Card.
|
Type
|
Description & Constraints

|

 SubstanceDefinition
SubstanceDefinition
|
|
|
SubstanceDefinition
|
|


 identifier
identifier
|
|
1.. |
|
|



 system
system
|
|
1.. |
|
|



 value
value
|
|
1.. |
|
|


 status
status
|
|
|
|
draft|active|retired|unknown |


 domain
domain
|
|
|
|
Binding:
Medicinal Product Domain
( example
)
|


 description
description
|
|
|
|
Textual description of the substance. |


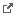 manufacturer
manufacturer
|
|
|
Reference
(Organization (ePI))
|
|


 molecularWeight
molecularWeight
|
|
|
|
|



 amount
amount
|
|
|
|
The molecular weight. |




 value
value
|
|
|
|
numerical value. |




 unit
unit
|
|
|
|
Unit of measure. |




 code
code
|
|
|
|
coded form of the unit of measure. |


 structure
structure
|
|
|
|
|



 molecularFormula
molecularFormula
|
|
|
|
Molecular formula (e.g. using the Hill system). |


 code
code
|
|
|
|
Codes associated with the substance. |



 code
code
|
|
|
|
Binding:
Substances
( example
)
|


 name
name
|
|
|
|
International Non-Proprietary Name (INN) of the substance; or United States Adopted Name (USAN) if applicable. |



 type
type
|
|
|
|
Name type e.g. 'scientific, 'brand' |
 Documentation for this format Documentation for this format
|


















































 Documentation for this format
Documentation for this format