| Name | Flags | Card. | Type |
Description & Constraints

|
|---|---|---|---|---|

|
Ingredient | |||


|
Reference (Processing Profile - Medicinal Product | Processing Profile - Administrable Product | Processing Profile - Manufactured Item) | Reference to the medicinal product, pharmaceutical product and/or manufactured item where the ingredient is used | ||


|
Role of the ingredient. PPL data normally only includes active ingredients. | |||


|
Substance code from EMA SMS | |||



|
Binding: EMA SPOR SMS Substances ( example ) | |||



|
||||




|
Slice: Unordered, Open by type:$this | |||




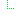
|
0..1 | Ratio | Strength per unit of presentation (10mg/vial or 10mg/0.5ml where 0.5ml is the size of the vial) | |




|
Slice: Unordered, Open by type:$this | |||




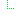
|
0..1 | Ratio | Strength per unit of measurement (20mg/1ml) | |




|
Strenth expressed in terms of a reference substance; reference strength type not distinguished. According to EMA IG, all products need to have reference strentgh (repeating the strentgh, if needed) | |||





|
Substance code from EMA SMS Binding: EMA SPOR SMS Substances ( example ) |
|||
 Documentation for this format Documentation for this format
|
||||