| Name | Flags | Card. | Type |
Description & Constraints

|
|---|---|---|---|---|

|
CDAR2.ManufacturedProduct |
XML Namespace:
urn:hl7-org:v3 This logical model cannot be the target of a reference |
||


|
1..1 | |||


|
1 .. | Slice: Unordered, Open by value:root, value:extension | ||


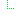
|
1..1 | |||



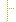
|
1..1 | Required Pattern: 2.16.840.1.113883.10.20.22.4.23 | ||



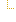
|
1..1 | Required Pattern: 2014-06-09 | ||


|
0..* | |||


|
1..1 | A medication should be recorded as a pre-coordinated ingredient + strength + dose form (e.g., “metoprolol 25mg tablet”, “amoxicillin 400mg/5mL suspension”) where possible. This includes RxNorm codes whose Term Type is SCD (semantic clinical drug), SBD (semantic brand drug), GPCK (generic pack), BPCK (brand pack). | ||



|
1..1 | Binding: Medication Clinical Drug ( required ) | ||




|
0..* |
Binding:
Clinical Substance
( example
) |
||


|
0..1 | |||
 Documentation for this format Documentation for this format
|
||||